The working principle of lithium battery
Lithium metal batteries generally use manganese dioxide as the positive electrode material, metal lithium or its alloy metal as the negative electrode material, and use a non-aqueous electrolyte solution.
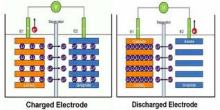
Basic principles of lithium batteries
Discharge reaction: Li+MnO2=LiMnO2
Total reaction of rechargeable battery:LiCoO2+6C = Li(1-x)CoO2+LixC6
Positive electrode material: There are many available cathode materials, and most mainstream products use lithium iron phosphate. Comparison of different cathode materials:
|
LiCoO2
|
3.7 V
|
140 mAh/g
|
|
Li2Mn2O4
|
4.0 V
|
100 mAh/g
|
|
LiFePO4
|
3.3 V
|
100 mAh/g
|
|
Li2FePO4F
|
3.6 V
|
115 mAh/g
|
Positive electrode reaction: Lithium ions are intercalated during discharge, and lithium ions are deintercalated during charging. When charging: LiFePO4 → Li1-xFePO4 + xLi+ + xe- When discharging: Li1-xFePO4 + xLi+ + xe- → LiFePO4.
Negative reaction: Lithium ions are deintercalated during discharge, and lithium ions are intercalated during charging.




 中文
中文 English
English